This week in the lab we are collecting our dandelion project samples that will be sent off to NC State to be run through a MiSeq machine to sequence DNA! The last two weeks we have been practicing our skills and getting ready for this moment!
We started off the week working with our 39 soil samples. We have planted our dandelions in triplicate, which means that we have planted 3 dandelions in 3 separate planters for each of the 3 soil samples. We have also put soil into a planter without adding a dandelion for a control. Each of the three teachers brought in 3 soil samples, so that leads to having 12 samples for each teacher. Then we collected soil from Prairie Ridge and kept 3 samples from there.
In order to collect the bacteria and fungus DNA we pulled up soil samples .1 and .2 and scrapped .25g of the soil that was attached to the root of the dandelion. Then on soil sample .3 we did not disturb the dandelion as much, because we just got close to the root without pulling the plant out of the soil. This trial is called method validation to see if we can get accurate data without uprooting the dandelion. In sample .4 the soil was just collected, because no dandelions were present. Each of the dandelions were then replanted in the soil and put back under the grow light and watered to continue growing for the next 3 weeks in the lab.
Me – scrapping soil off of the dandelion tap root.
Show an awesome dandelion taproot and rhizosphere!
Next we began to extract the DNA from all of our samples. We completed 2/3rds of the extractions on Monday and then finished up on Tuesday. After we completed the extraction we got to use the nanodropper to see if we successfully extracted the DNA before we put it into the PCR machine to duplicate the DNA. We were all a bit nervous, because this was the real experiment and not just practice any more. We were all very excited when the nanodrop showed all of our samples had a very nice concentration of DNA, so we set up the samples and ran the samples through the PCR machine.
The cool part of setting up these samples was that we were using a new “Master Mix” that cut out some of the pipetting steps. We also were adding in adaptive primers to allow our DNA samples to be sequenced in a high tech DNA sequencing machine called a MiSeq. This machine is housed at NC State and will allow the genomics lab to create 15 million copies of DNA in one run! This technology is amazing, and only costs about a thousand dollars. This is comparatively inexpensive when compared to just a few years ago where labs would have to spend tens of thousands of dollars or hundreds of thousands of dollars to duplicate DNA in much smaller quantities. Team Dirt is on the cutting edge!!!
 Dr. Urban, Dr. Horvath, and Dr. Stevens as they help us through our DNA extraction.
Dr. Urban, Dr. Horvath, and Dr. Stevens as they help us through our DNA extraction.
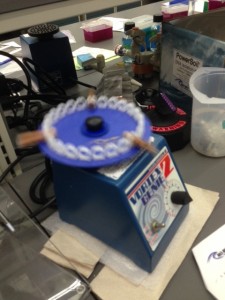 Using the Vortex machine to begin extraction of DNA.
Using the Vortex machine to begin extraction of DNA.
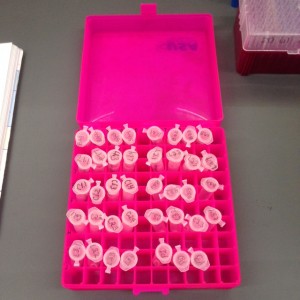 Our 39 samples of extracted DNA.
Our 39 samples of extracted DNA.
We were all very excited to see our electrophoresis gel after such a success on nandrop, but alas……our gel showed that the DNA was not duplicated correctly. All 4 of us on Team Dirt were very sad, but we continued on. We knew that failure is what science is all about! We learn from our failures, and that is what we did.
Dr. Stevens ran a short series of trouble shooting experiments with our extracted DNA. She picked 5 random samples out of our 39 and then retested them 4 ways. The first two trials used the “Master Mix”. Trial 1 used the MiSeq adapter primers, Trial 2 used the non-adapter primers. Trials 3 and 4 did not use the “Master Mix” and instead each individual ingredient, including the Taq was added by hand. Then Trial 3 used non-adapter primers and Trial 4 used adapter primers. After these trouble shooting experiments were run, and we got to look at the gel, we saw that the “Master Mix” did not work very well with our soil DNA, but that our hand made mix was giving us very good readings. This made all of us on Team Dirt very very happy! We now know that we can take all 39 samples of soil DNA and remix them and prepare them for PCR machine and being shipped off to NC State for the the MiSeq machine!!!!
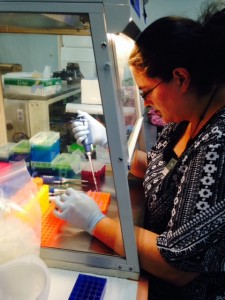
Me working on setting up PCR.
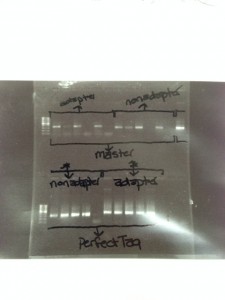 Our Trouble Shooting Electrophoresis Gel
Our Trouble Shooting Electrophoresis Gel
Today our team also plated soil extracted from all .3 plants on various agar. We have our experimental soil extracted agar plates that we created last week, as well as nutrient agar and bright pink fungal plates! We are going to grow the bacteria and fungus on these plates and take pictures of the growth each day, so that students can visually see species diversity and how it can grow and change over time.
This week Team Dirt has also been working on building our curriculum from the ground up. It has been challenging, but we are determined to build a fantastic PBL lesson that will be and excellent Citizen Science project.
Random Shots from the Week

 A storm approaching and reaching my 17th floor room.
A storm approaching and reaching my 17th floor room.
 The Biodiversity lab preserved multiple animals from collections in the lab this week. This is a chipmunk above.
The Biodiversity lab preserved multiple animals from collections in the lab this week. This is a chipmunk above.